In this article, I want to discuss the most mind-blowing scientific video I have ever seen. On Veritasium, Derek Muller explored the mystery of tree height:
The first time I saw this video, I was speechless… and confused! There is an awful lot of information in this video, as it mentions plenty of physical phenomena. So let’s explore them in greater details!
Water Height
Hummm… I could. But Derek did it himself very well in a previous video:
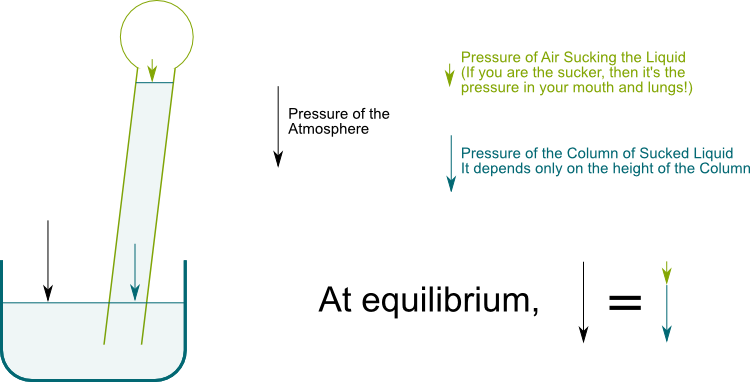
Hummm… We’ll get to this later. For now, what’s of interest to us is the vertical top to down pressure on the top surface of fluids. This vertical pressure is the cumulated weight of the layers of fluids above. Imagine you were pressing vertically some volume of water. For the surface of the water to be horizontal, we need an equal vertical pressure on every point of the surface. The following figure displays the pressure on two point of a horizontal surface of the water when a straw sucks.
The soil is filled with water. The surface of soil is under atmospheric pressure, while the tree is like a straw which sucks water.
When you suck in a straw, you are actually increasing the volume of your lungs. The air pressure of your mouth and lungs thus decreases, which enables water to go up. But this air pressure cannot be negative. Thus, at equilibrium, the water column pressure in blue cannot exceed the atmospheric pressure in black. This water column pressure exceeds the atmospheric pressure if the water column height exceeds 10 meters.
Yes! And, in California, sequoias can even be a hundred of meters high!
Indeed they do. But before getting to this, let’s talk about the different refuted hypotheses of Derek’s video. Although they don’t turn out to be correct, they involve physical properties which are essential to explain other phenomena, and have great applications.
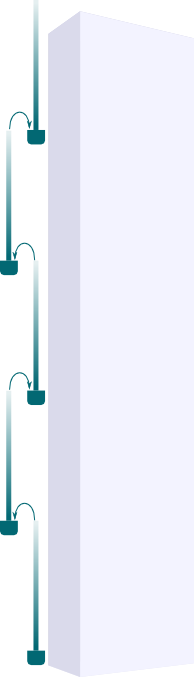
Yes! It’s a natural idea to come up with as it’s what’s used in skyscrapers to pump water to top floors, as displayed in the figure on the right. With this setting, water can first be sucked through the first column, then poured in the second one, then sucked again… and so on to the top floor. But as Derek said, researchers have shown that this was not what happened.
Osmosis
The second wrong explanation mentioned by Derek is osmosis.
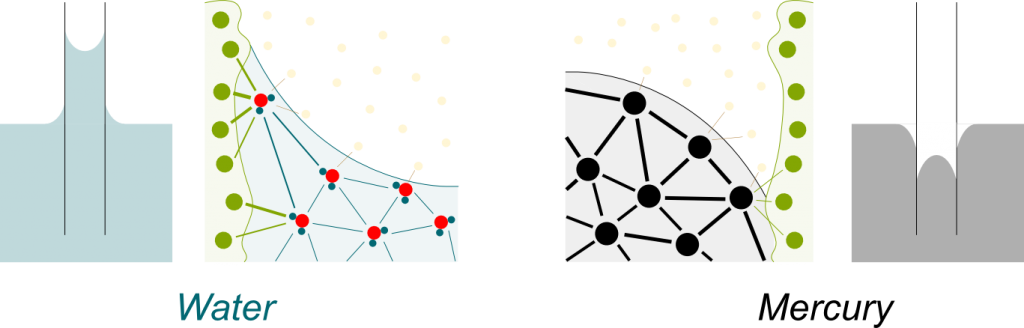
Osmosis is a phenomenon which occurs when two solutions are separated by a semi-permeable membrane through which only water goes. Other particles, called solutes, can’t. Then, there is a natural tendency of water to go to from the less concentrated solution to the more concentrated one.
Solute particles attract water molecules. They sort of glue a few water molecules around them. This prevents these glued water molecules from going through the semi-permeable membrane. Now, only unglued water molecules can go through the membrane. These unglued water molecules are called free water molecules. The greater the concentration, the fewer free water molecules there are. This is illustrated below, where solute particles are the bigger green dots, glued water molecules are small green dots and free water molecules are small blue dots:
Yes it does! Basically, each free water molecule goes through the membrane with a certain probability. Yet, since there are many more free water molecules on the right, then there are many more free water molecules moving from right to left than from left to right. Thus, in average, the net flow is a flow of water from right to left. This phenomenon is called diffusion. It is similar to the reason why milk gets spread when poured in a cup of coffee.
Yes. At the membrane location, the net flow of free water molecules behaves like an added pressure of the less concentrated solution on the more concentrated one. So, if the soil was a less concentrated solution than the water in the xylem tube, which transports water up in trees, then it could press the water up. But, as Derek pointed it out, in many areas, this is not the case.
Actually, no, I’m not going to mention capillarity just now. It’s a major feature of trees and I’ll get to this later.
Negative Pressure
For water to be sucked up, there must be a pressure difference between the top and the bottom of the tree. Instead of sucking water by decreasing air pressure on top of the column, we can increase pressure at the bottom. This can be done by pressing the liquid, just like you can get toothpaste out of its container.
We just need to create a water pressure of 2 atmospheres at ground level.
Yes… But a 10 atmosphere pressure is enormous! It can break containers, which can be very dangerous. Plus, there is no mechanism in trees which could enable such pressure. Instead, as explained by Derek, trees leave the pressure at ground level at atmospheric pressure and create negative pressure in the xylem tube.
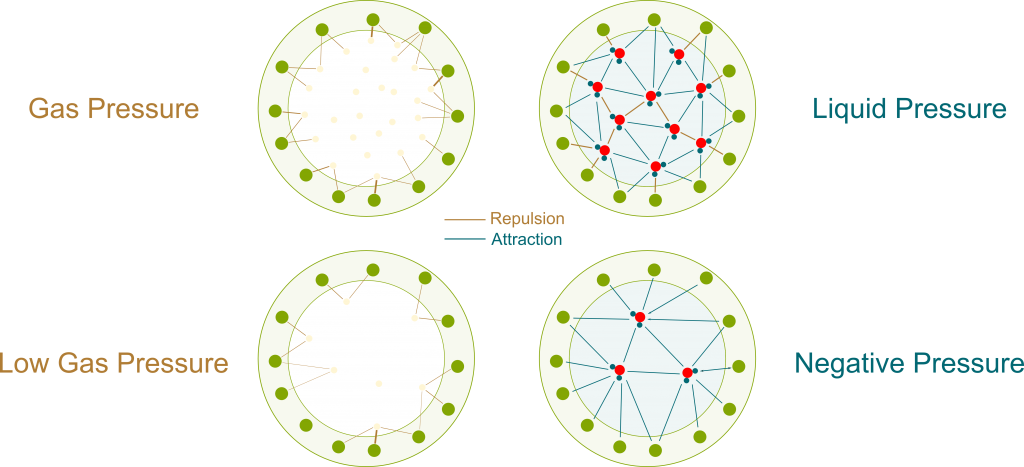
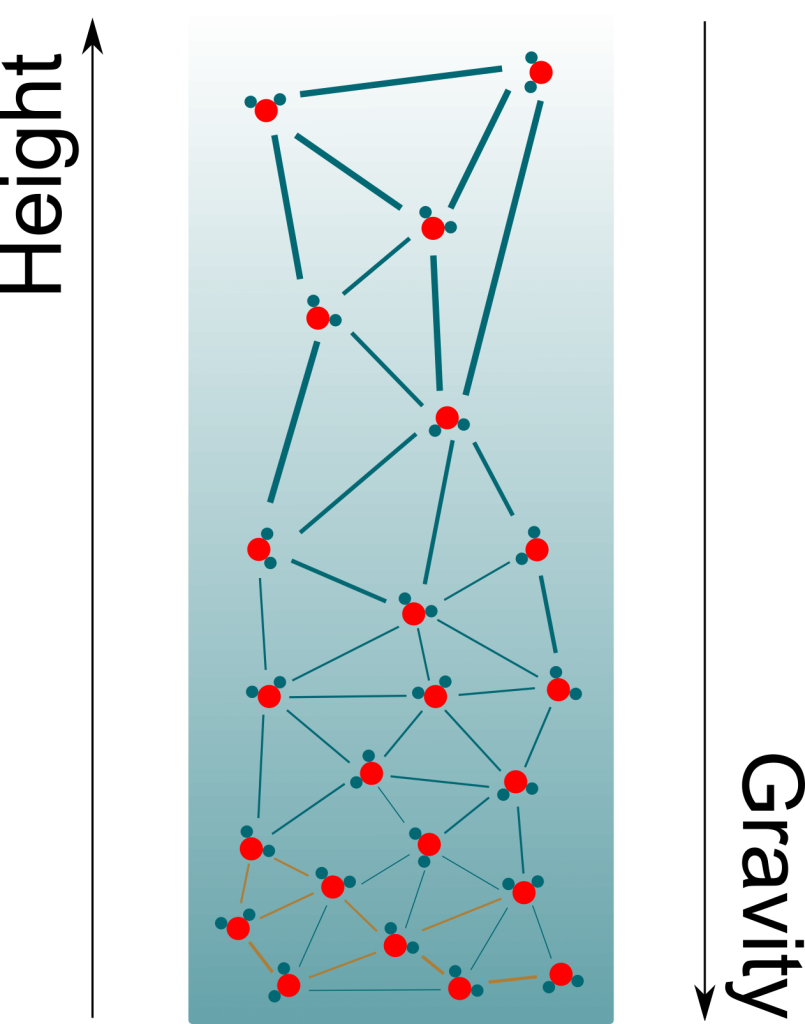
As opposed to gas molecules, water molecules don’t only interact by collisions. There are other forces between them, which can be electrostatic forces or Van der Waals forces.
These other forces can be repulsive when two molecules are too close to each other. But what’s of greater interest to us is that they become attractive for molecules which are farther apart than they should be. This pull means that a great force is necessary to extend a liquid. If we do extend a liquid inside a container, then the water molecules would be pulling on each other as well as pulling the container itself. This is displayed in the following figure, where bolder links are stronger forces.
Pressure is usually taught as the effect of collisions of particles. This effect is only repulsive. Thus, the pressure associated with collisions is always positive. But since the pull of liquids displayed above has all the properties of pressure (except being a push), we can associate it with a pressure, just like we associated osmosis with osmotic pressure. And because it’s a pull rather than a push, it is negative.
The figure only displays a few particles. In reality, there are billions of billions of them. Plus, they are all in motions at all time. Even though the exact pressure may differ depending on the location of particles, the differences are so small that they just can’t be observed. At our scale, it’s a extremely good approximation to consider that pressure is uniform.
Precisely! As you can see on the figure above, negative pressure implies that water molecules suck each other. More precisely, the more negative the pressure is, the more water molecules suck. By having more negative pressures at their tops, trees manage to suck water from the soil to their leaves.
Gravity! Basically, what trees do is hold on to water at the top of the xylem tube. Gravity then brings down most of the molecules. Only a few molecules remain on the top of the trees, hence creating immense negative pressures. At equilibrium, the variation of pressure compensates gravity. This corresponds to a pressure decrease of one atmospheric pressure every ten meters.
The man surely knows what he’s talking about! The greatest visualization of this phenomenon is by observing a slinky. The way a slinky falls is mesmerizing, but what I want you to notice is how stretched by gravity it is when someone holds its top:
Yes! The tension gets much stronger at the top, because gravity sorts of pulls most of the rings down. Similarly, the tension, or, as it’s rather called, the negative pressure in the water column is much stronger at the top.
Almost! They have capillarity.
Capillarity
Capillarity is an ability of some liquids to climb some containers despite gravity.
Liquid molecules climb containers when the attraction between liquid molecules and container molecules is greater than the attraction between two liquid molecules. As liquid molecules are in motion, some go up and find themselves trapped by the attraction forces with container molecules. They then pull up other liquid molecules around.
Yes. On the opposite, when the attraction between liquid molecules is greater than with container molecules, the liquid goes down, as is the case for mercury:
If the surface of the solid was perfectly flat, then the total attraction of the container on the water molecules would be pointing left. But the surface isn’t flat, and its irregularity implies that, at some position, the total attraction does point upwards, which explains that water does climb containers.
As you can imagine when watching at the figure above, a column of water still has to face atmospheric pressure. Now, the larger the tube, the greater the ratio of water molecules which face atmospheric pressure is. The xylem tube is too large to get water up more than 1 meter. But the tube ends with a gigantic number of extremely thin pores in leafs, at the ends of stomata (plural for stoma). These pores are called cell wall pores. While the xylem tube is 20 to 200 micrometers large, the cell wall pores are 2 to 5 nanometers large. This is nearly the scale of molecules and means that the surface of the meniscus is only made of a few hundred molecules!
Yes! The width of the pores is 10,000 times less than the xylem tube. Yet, the height capillarity can suck is inversely proportional to the width of the tube. Thus, the pores can withstand a water column which is 10,000 times taller than the xylem tube! Since the xylem tube could suck water 1 meter up, the pores can suck water kilometers up!
In these smaller cases, inter-molecular interactions get stronger than other forces like gravity. This has counter-intuitive consequences like surface tension. For instance, water behaves nearly like solid matter for ants (check it out on google image!). The most stunning illustration of surface tension is probably what happens in zero gravity, as experimented by CSA Astronaut Chris Hadfield:
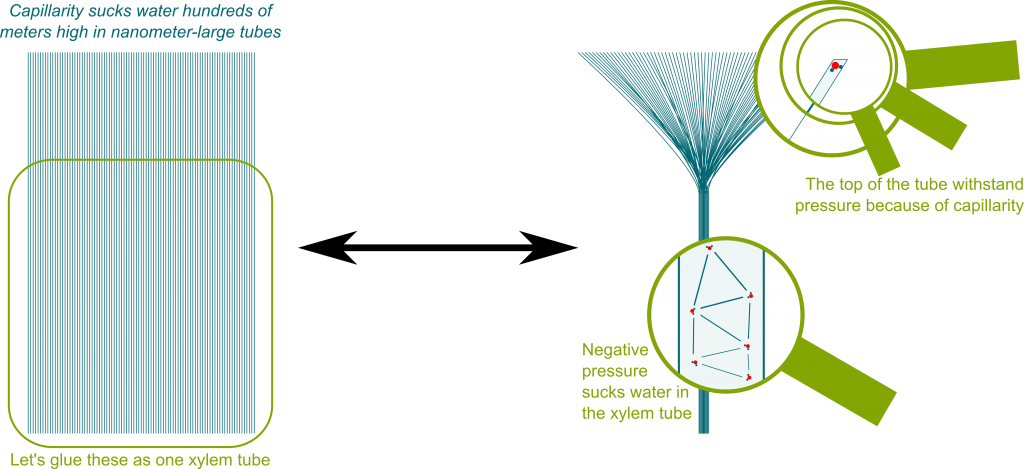
Precisely! Since there are billions of pores on a leaf, and nearly a million leaf on a tree, this means that a tree has about millions of billions of tiny water-air interfaces. In fact, the xylem tube can be thought as a union of tiny tubes which each correspond to a pore. Each of these tubes is so tiny that it sucks water up hundreds of meters by capillarity (and could suck even more!). This is displayed in the figure below:
My mathematical side wants to highlight that trees need to keep this feature as they grow up. Fortunately, very basic rules of branching and growing enable them to develop their mesmerizing structure. This basic process is a fractal dynamic system. Learn about fractals with Thomas’ article and with Scott’s first and second articles on dynamic systems
.
By evapotranspiration!
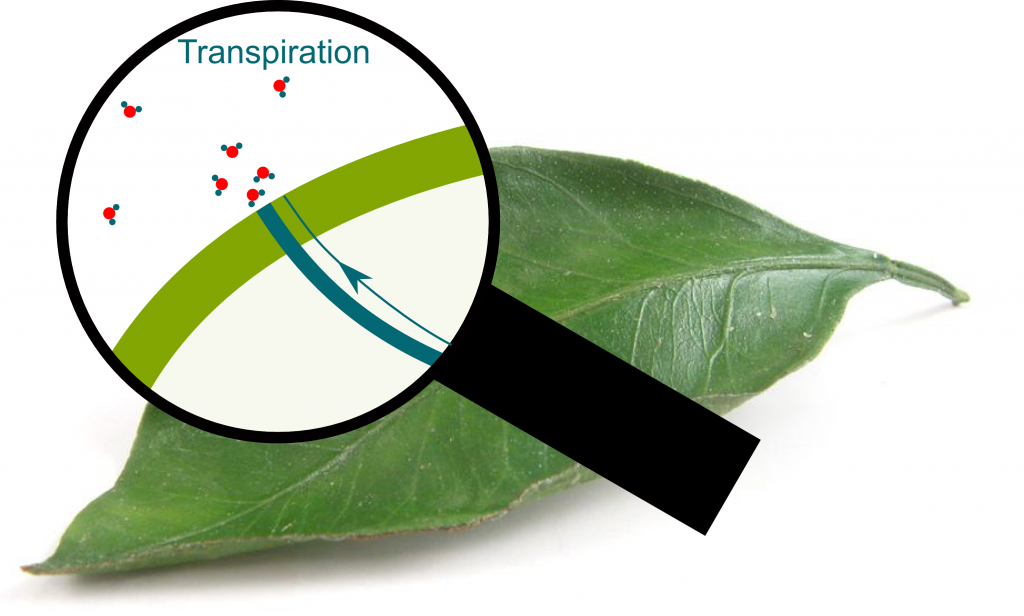
Transpiration
Yes. Stomata can be closed and maintain the equilibrium, or open and let the water column be in contact with atmospheric air. Evaporation then occurs and enables some water molecules escape the water column.
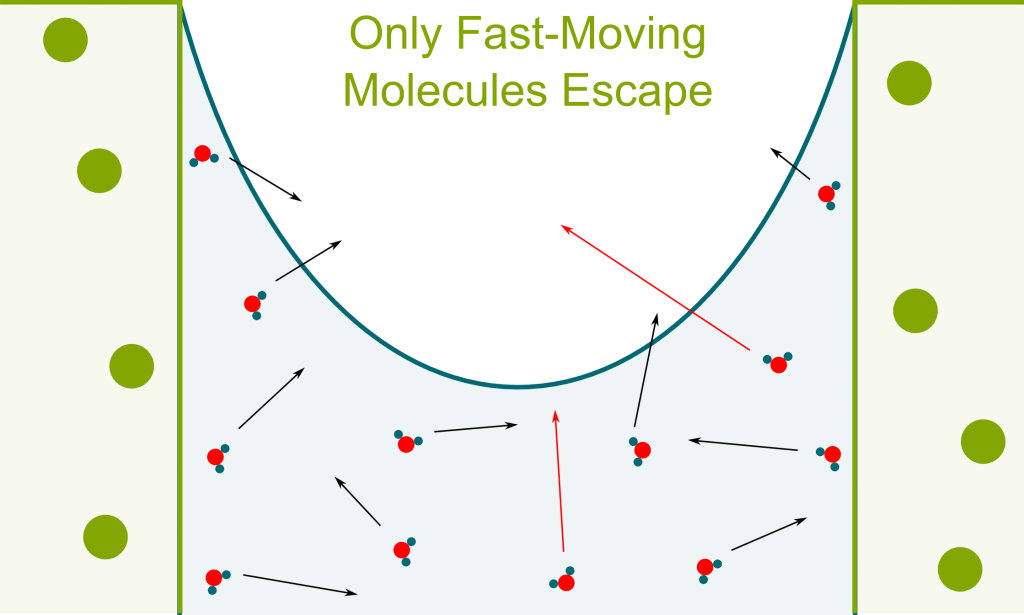
In liquids, molecules move at different speeds. Most don’t have enough speed to escape the attraction forces with other liquid molecules. However, after random sequences of collisions, some liquid molecules will reach speeds great enough to escape the attraction forces. As they break the links with other molecules, they turn into gas phase.
Yes! Because fast molecules end up escaping, only slow molecules are left. Since the temperature is related to the average speed of molecules, the temperature of molecules which are left decreases. Thus, transpiration enables cooling. This idea is the basis of magnetic cooling which has been essential in approaching absolute zero. Learn more with my article on the frontier of cold!
As trees sweat, they lose molecules. The density of molecules at stomata thus decreases. This lowers the pressure into even more negative one. The whole water column then gets sucked, which enables water to go up the tree. In other words, transpiration is the mechanism which stretches water on the top of trees, and enables water to move up the tree.
Let’s get back to the slinky. Imagine you had an extremely long one that touches the ground. Then, as you move it up, holding it up high, the slinky will move up, because the tension at each point of the slinky increases and pulls it up!
A Conclusion with Open Questions
The physics of water in trees is so much more complicated than one would expect. I hope Derek’s videos and my article have amazed you by the numerous simple puzzles this physics poses and helped you better understand some of the mysteries! Now, truth is, there is still a lot I don’t get… and I count on you to help me out. This conclusion may sound despairing, but puzzling unanswered mysteries are actually the great intrigue of science. And the greatest part of science is trying to figuring it out, together. So, please, help me by sending me explanations or links!
First, I have trouble with the concept of activation energy, especially on a molecular level. I guess that water in the xylem tube can’t boil because it is surrounded by other water molecules and thus can’t escape attraction forces, as it would do by entering some bubbles. But I’d love confirmation. I don’t understand either how the xylem tube manages to be filled with water only. Surely, there must be air in the soil, but, apparently, it doesn’t get sucked. Why? Apparently, it’s because of the membranes water has to go through to reach the xylem tube. These membranes manage to filter water and leave out air molecules.
Yes. Another phenomenon mentioned in Derek’s video is absorption of carbon dioxide at stomata thanks to transpiration. However, I don’t understand why transpiration enables catching carbon dioxide molecules. Is it related to diffusion or negative pressure? Can’t gas molecules go down the xylem tube and make it boil? Why not?
Yes, there’s one final major trouble. I wonder what the pressure of water inside tree cells is. Is it at the same pressure as water in the xylem tube? If it isn’t, how does water go from the xylem tube into cells? Or, more precisely, how does water not go from the cells into the xylem tube? If it is, shouldn’t leafs be stretched as we take them off the tree and pressure inside increases, especially if we put them in water at atmospheric pressure?
Wait! It’s not over! Instead of answers, you guys have given even more amazing questions! In particular, Edouard Yin has raised some particular intriguing ones! What happens in winter as leafs fall? Does water fall down too? How does it get suck back up in spring? Is there some mechanism which first grows leafs at the bottom? I hope these questions will be troubling you! But I’ll also leave you with another amazing fact about trees and water, with this awesome video by MinuteEarth:






Informative article, exactly what I wanted to find.|
Technically, I really feel I can say ‘we’ as a result of after sufficient whining, begging and tears (if need be), my tech-savvy, yet blog-weary husband comes to my
rescue for all things WordPress, podcast and ethical assist.